FEBRUARY 2023
BALANCING THE BENEFITS OF OPIOIDS
Table of Contents
OPIOID PRESCRIBING LIABILITY REMAINS TOP NEWS
Physicians licensed to prescribe opioid-based medication for pain management walk a careful tightrope—remaining constantly vigilant to adhering to state and federal guidelines, managing their patients’ symptoms, and avoiding long-term dependency concerns. This prescribing environment is rife with areas of medical liability concern.
Some 20 states require opioid/controlled substance or pain management courses as part of their continuing medical education (CME). In some cases, this is required of all practicing physicians, though some states only have educational requirements for those holding a Drug Enforcement Administration license to prescribe controlled substances.
As with any topic of particular importance to the healthcare community, we strive to provide ample educational resources. Some are intended to assist our insureds in navigating mitigating the risk in their practice, while others focus on keeping you, our agency partners, aware of key developments so you can incorporate them into your sales conversations.
A highlight of this issue will be insight from John Bowman, founder and Chief Executive Officer of Sure Med Compliance. He is considered a national key opinion leader in matters of liability and medicolegal risk in pain management settings. The Sure Med Compliance digital health platform is scientifically validated to mitigate risk to both patients and providers in pain management settings. ProAssurance has an exclusive affiliation with Sure Med, which aligns with our efforts to provide risk-reduction tools and strategies to our insureds.
We will also share insight from our own Risk Management team, as well as key developments in the healthcare industry that impact your clients. Our team is always available to answer specific questions.
WHEN A GUIDELINE BECOMES A RULE
HOW THE NEW CDC GUIDELINE FOR PRESCRIBING OPIOIDS WILL AFFECT
PATIENTS AND PROVIDERS IN PAIN MANAGEMENT SETTINGS
With the release of the revised Centers for Disease Control and Prevention (CDC) Clinical Practice Guideline for Prescribing Opioids for Pain, many chronic pain patients and healthcare providers dared to breathe a sigh of relief. For years they had suffered negative consequences stemming from the initial version of this guideline, and they have been eagerly awaiting its updated recommendations. In the wake of the 2016 guideline, many patients began the devasting endeavor of being aggressively tapered, abruptly discontinued, and even completely dismissed from their pain clinic. This was a death sentence for many, as they quickly learned the horror of opioid withdrawal, driving them to experiment with illicit drugs in search of relief. Physicians, on the other hand, were either criminally prosecuted, lost their state license to practice medicine, or both.
The new guideline update contains important changes, most notably the relaxing of strict morphine milligram equivalent (MME) limitations in favor of a more individualized approach to treatment. And while it may appear as if common sense has prevailed and the overcorrection has, itself, been corrected, prescribers and patients shouldn’t break out the champagne just yet.
This updated guideline, while an improvement from the last, will not affect the immediate change that most in the pain community are hoping for. To understand why, it’s important to first understand the difference between a “guideline” and a “rule.”
BACKGROUND
The CDC’s 2016 guideline on opioid prescribing was created in response to the unfolding opioid epidemic, one whose characteristics were quite different from the current situation we are in now. In the years leading up to this federal response, most opioid-related overdoses involved prescription opioids in combination with commonly co-prescribed sedatives such as benzodiazepines. These highly potent medications were being prescribed to patients, some with risk factors (that went undetected due to the time constraints of an office visit) who ultimately developed substance use disorders. Once in the throes of addiction, many patients turned to illicit drugs such as heroin to offset the effects of tolerance, which often accompanies chronic opioid use.
Given the well documented correlation between nonmedical opioid use, heroin use, and overdoses, the CDC’s main goal of reducing the number of overdoses by reducing the number of opioids being prescribed made perfect sense. Their modus operandi: a guideline.
A guideline is defined as “a general rule, principle, or piece of advice.”
In the civil liability world of expert witnesses and “reasonable prudent physician” tests, “advice” may be a powerful tool or tactic to sway a judge or jury. In the criminal and regulatory world, however, “advice” hardly sounds like an authoritative doctrine that must be followed religiously. So how did a well intentioned gift from the government become a curse?
While CDC was busy crafting a guideline, state board members in 48 states were scrambling to follow New York and Washington, both of which had officially published “rules” defining the phrase “legitimate medical purpose” as it relates to opioid prescribing.
Legitimate medical purpose comes directly from the Code of Federal Regulations and is the regulatory and civil standard that is applied in misprescribing cases. Their reason for promulgating these rules was simple: only state licensing boards and legislatures have the authority to define what constitutes the legitimate medical purpose of opioids.
A rule is defined as “one of a set of explicit or understood regulations or principles governing conduct within a particular activity or sphere.”
A few key words—“explicit,” “regulation,” and “governing”—should invoke the true essence of the word “rule” and how starkly different it is from “guideline.” But how exactly did a guideline that was intended to be advice turn into a rule that could be used to criminally prosecute and strip a physician of their state medical license?
By 2017, most state licensing boards had at least some language containing the phrase legitimate medical purpose. This language, relegated to overly vague, noncommittal verbiage, encouraged physicians to follow state and federal law—a subtle reference to the Controlled Substance Act of 1970 and included a link to the “Federation of State Medical Boards (FSMB) Model Policy on the Use of Opioid Analgesics in the Treatment of Chronic Pain, 2013.”
What they did not have was an actual set of rules defining how and when a physician should initiate or continue opioid therapy. In light of this deficiency, many state boards looked no further than the CDC guideline to inform their definition of legitimate medical purpose. What ensued was an exercise in corner cutting that would make the most shameless plagiarist blush.
With a general acceptance that the CDC guideline was authoritative and comprehensive enough to use as a template, state boards began publishing rules nearly identical to the CDC guideline. This transformation from a comprehensive document filled with good advice to an overly complicated, authoritative standard not even the most dedicated clinician could meet was the proverbial nail in the coffin for patients and providers in the pain space.
So how has this strategy on curbing the opioid epidemic turned out? Between the years 2016 and 2022, there has been a widening inverse relationship between prescription opioids and overdose deaths.
In short, the plan to save lives has actually resulted in more deaths.
WHERE WE ARE TODAY
Welcome to 2023. The leading cause of all overdose deaths is illegally manufactured fentanyl. Overregulation and overcriminalization of pain doctors, due to the rigid interpretation of the 2106 CDC guidelines, have created real barriers to patient access. Those patients, desperate for medical care, are finding themselves with only one option: having their healthcare delivered by friends, family, and drug dealers. The evolution of this epidemic, coupled with the failed policies that have exacerbated it, finally motivated the CDC to reevaluate some of their recommendations.
And now that the long-anticipated update to the CDC guideline has finally been published, what does it mean? Well, as you can guess, it doesn’t mean much just yet. We’ll see real change when the state boards update their rules. (To date, only one state has actually done so—Rhode Island.) The changes that providers and patients in the pain community are hoping for are still to come, but this is likely to be a long process.
TECH-ENABLED SOLUTIONS
In the meantime, for physicians who prescribe opioids to truly mitigate risk, they must standardize how they determine and document the rules and requirements. This means capturing up to 26 data points per patient encounter and showing clear evidence of following published treatment protocols. Using tech-enabled solutions such as the Perspectives in CareSM digital health platform can help ensure the appropriate chart documentation is always present and can save clinicians their most valuable asset: time.
We hope that you’ll share this important information with your clients working in pain management settings. If you have questions or would like information on how ProAssurance and Sure Med can help your clients with safer prescribing practices, please reach out.

PARTNERING WITH SURE MED COMPLIANCE FOR RESPONSIBLE PRESCRIBING
AT THE FOREFRONT OF OVERDOSE PREVENTION
It can be a difficult balance treating patients’ pain while also taking crucial steps to avoid misuse, abuse, dependence, and overdose of opioids. Providers must recognize “red flags” and signals of misuse, abuse, or dependence. In the context of treatment for chronic pain, addictive behaviors may be difficult to identify. That’s why we’ve partnered with Sure Med Compliance. Their mission is to end the overdose epidemic through greater prescribing compliance by offering the first and only digital health platform scientifically validated to mitigate risk to both patient and provider in pain management settings.
By delivering clinical insights that help influence safer prescribing decisions, Sure Med helps protect healthcare providers from liability and allows doctors to create safer exposures to controlled substances.
They recognized a point-of-care problem: To initiate or continue controlled substance therapy legally, a physician must determine and document up to 26 data points in a 5- to 10-minute office visit. This may not be feasible for busy doctors, so it may not get done. The unintended consequences may lead to poor outcomes and provider liability.
With Sure Med’s digital health platform, the patient is evaluated electronically, and the results are delivered immediately to their Electronic Health Record. This eliminates 80% of the time needed to determine and document the necessary data points to initiate or continue controlled substance therapy.

Through our exclusive affiliation with Sure Med, ProAssurance is at the forefront of helping physicians to prescribe more responsibly, leading to better outcomes for patients. We encourage your clients who regularly prescribe opioids, especially those who prescribe for chronic pain, to learn how Sure Med can help them develop and maintain safe and responsible prescribing practices, which in turn, should lead to better outcomes for their patients.
By partnering with companies dedicated to new technologies and innovation that benefit patients and improve lives, ProAssurance is able to facilitate reducing provider risk and ultimately protect others. It is an intersection of potential that aligns with our purpose.
Learn More: SureMedCompliance.com
RISK MANAGEMENT OPIOID RESOURCES
As Sure Med Compliance works to help physicians and their facilities and organizations manage and track information regarding opioid prescriptions, ProAssurance works to provide insureds with educational materials. These materials range from short videos that provide advice from a subject matter expert to full seminars that help physicians earn their continuing medical education (CME) credit. These resources also help adhere to some 20 states’ requirements for a CME credit relating to the prescription of controlled substances and/or pain management. The ProAssurance Risk Management team also provides samples of different documents physicians may come across when prescribing opioids as well as recommended external resources.
SEMINARS
The ProAssurance Risk Management team offers several seminars focusing on prescribing opioids, including keeping a physician’s patient safe, risk management regarding opioids, and the most recent CDC guidelines for prescribing opioids for chronic pain. These seminars take a longer amount of time, but physicians can earn CME credits once they successfully complete a seminar and pass the posttest with a score of 75% or greater. All the seminars take roughly an hour to complete, and they are located on Risk Management’s seminar page.


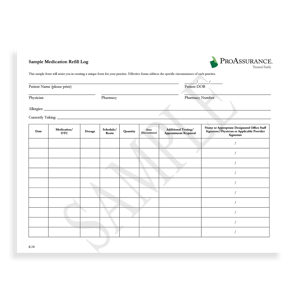
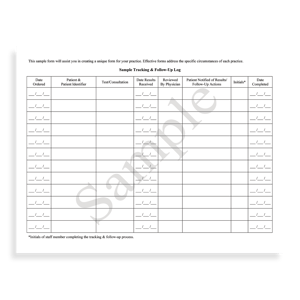
SAMPLE FORMS
There are sample forms that physicians may use when prescribing opioids for chronic pain management. Those forms include medication refill guidelines, medication refill log forms, and medication education forms for patients. Those forms are also found on Risk Management’s site.
TWO-MINUTE VIDEOS: WHAT'S THE RISK?
These videos are overviews of topics that a physician and staff can watch in a short period of time. The two-minute video “The Drug Seeking Patient” provides an educational opportunity for physicians to determine whether a patient is seeking unnecessary medication or has a legitimate pain management issue.
The ProAssurance Risk Management team also recommends external resources to organizations like the National Institute on Drug Abuse.
OPIOID PRESCRIBING
The Tragic Toll of Opioid Abuse in America
In 2020 (the latest year for which CDC data are available), 16,416 Americans died of prescription opioid-involved overdose — a 16% increase over 2019 and the first increase since 2016.1,* This despite decreases in both total prescriptions (-6.8%) and prescribing rate (-3.4 points).2,† It’s no surprise, then, that overdose death involving opioids is referred to as a public health emergency and an epidemic.3
Although the death rates‡ for prescription opioid overdose are lower than death rates for synthetic opioids — over which healthcare providers have little direct influence — research seems to indicate that some patients who abuse prescription opioids will at some point transition to illicit opioids such as fentanyl and heroin.4 Consequently, there are good reasons for opioid prescribers to remain vigilant about carefully balancing the risks and benefits of opioid therapy for chronic pain.
A review of our closed claims indicates that claims involving opioid therapy in chronic pain management patients generally involve overdose deaths. Allegations supporting negligence claims against physicians include excessive opioid prescribing; failure to refer the patient for pain management, addiction, or behavioral health treatment; failure to discover the patient was doctor shopping; failure to recognize suicide risk; and negligent tapering.


THE PATH FORWARD
The case studies in our Knowledge Library use closed claims, policyholder inquiries, and medical board actions to identify pain management best practices “that balance a focus on optimizing function, quality of life (QOL), and productivity while minimizing risks for opioid misuse and harm.”5
CONCERNS
- 21-29% of patients prescribed opioids for chronic pain misuse them.6
- 8-12% of patients prescribed opioids for chronic pain develop an opioid use disorder.6
CHALLENGES
- Clinicians are responsible for the safety of the patients for whom they prescribe opioid pain medications.
- There are no shortcuts to reducing the risk of accidental and intentional overdose.
- Misinterpreted and misapplied prescribing guidelines can cause patients to turn to illicit drugs like fentanyl or heroin to manage their pain and withdrawal symptoms.5
STRATEGIES
- Develop pain treatment plans that balance optimizing function, quality of life (QOL), and productivity while minimizing risks for misuse and harm.
For more information and links to case studies and risk reduction strategies—as well as notes and numbered references—visit our Knowledge Library article “Opioid Prescribing for Chronic Pain: Case Studies and Best Practices.”

SCY: A TECHNIQUE TO OPEN CRITICAL CONVERSATIONS
 As the opioid epidemic continues to plague the healthcare industry, it presents a unique challenge for healthcare professionals, particularly physicians, who are often torn between their duty to alleviate pain and the risk of legal consequences, even imprisonment. It also creates a challenge for MPL agents who recognize the importance of including discussions about opioid liability as a regular part of sales conversations with prospects and clients. Opioids are a hot topic with physicians and will likely get their attention. The challenge is that doctors often avoid or dismiss discussions they perceive as sales pitches, especially if they believe they’re already managing the risks involved.
As the opioid epidemic continues to plague the healthcare industry, it presents a unique challenge for healthcare professionals, particularly physicians, who are often torn between their duty to alleviate pain and the risk of legal consequences, even imprisonment. It also creates a challenge for MPL agents who recognize the importance of including discussions about opioid liability as a regular part of sales conversations with prospects and clients. Opioids are a hot topic with physicians and will likely get their attention. The challenge is that doctors often avoid or dismiss discussions they perceive as sales pitches, especially if they believe they’re already managing the risks involved.
For instance, if you try to initiate a conversation with a physician about the importance of liability coverage when prescribing opioids, the doctor may balk if they already follow the Centers for Disease Control's (CDC) Clinical Practice Guideline for Prescribing Opioids for Pain. They may also feel that their current medical professional liability (MPL) policy is sufficient. However, it can be more difficult for physicians to ignore an opportunity to learn how their colleagues manage similar risks. By opening a meeting with concrete examples and demonstrating your expertise, you may gain the physician’s attention and interest, and engage them in a meaningful discussion.
One of the best ways I’ve found to avoid the typical HCP response of "I'm happy with my current product” is by opening with a technique I call SCY that shifts the initial focus away from the sale.
SCY stands for Situation / Colleagues / You:
Situation: Describe a situation the prospect has or is likely to encounter.
Colleagues: Describe how the prospect’s medical colleagues commonly respond in that situation.
You: Ask the prospect, “What do YOU do in this situation?”
One key aspect of the SCY approach is that it centers the conversation on the physician's expertise and clinical approach. This helps to establish a more genuine and meaningful dialogue rather than simply trying to pitch a product or service. Here is an example of how the SCY method could open a discussion: "Prescribing opioids to patients with a history of abuse is a potential liability issue. Some of your internal medicine colleagues prescribe only the minimum dosage for the shortest duration, whereas others won’t prescribe opioids to these patients and refer them to a pain management specialist instead. What do you do in this situation?”
You'll initiate a more personalized conversation by introducing a specific circumstance and asking for the prospect's opinion instead of merely giving a product-focused sales pitch. The SCY approach can be particularly practical because it allows you to learn what is most important to the prospect in a given situation or context. When you transition to discussing appropriate strategies or coverage options, you can tailor the benefits you present to the prospect’s specific concerns. This is a more compelling approach than simply reciting a list of benefits and hoping that some of them resonate.
As the clinical and legal landscape around prescribing opioids evolves, you will have ongoing opportunities to provide value to physicians by sharing strategies for managing the risks associated with this class of drugs. The SCY technique can be a helpful tool for starting a personalized and comfortable conversation about the challenges of prescribing opioids and for helping doctors understand the importance of staying current with best practices and obtaining adequate (MPL) coverage.

Written by Mace Horoff of Medical Sales Performance
Mace Horoff is a representative of Sales Pilot. He helps sales teams and individual representatives who sell medical devices, pharmaceuticals, biotechnology, healthcare services, and other healthcare-related products to sell more and earn more by employing a specialized healthcare system.
Have a topic you’d like to see covered? Email your suggestions to AskMarketing@ProAssurance.com.
.png?width=300&name=MicrosoftTeams-image%20(28).png)
Unlike methadone, the traditional medication to wean people off heroin, or other opioids, buprenorphine can be prescribed at primary care clinics and dispensed at neighborhood pharmacies. Federal and state authorities have encouraged more front-line health care professionals to prescribe Suboxone (buprenorphine and naloxone) and other medications containing buprenorphine for patients trying to overcome opioid addiction. Federal regulators have made it easier for doctors, NPs, and PAs to become certified to offer the service.
A crackdown by U.S. drug wholesalers in response to the opioid crisis is preventing some pharmacists from dispensing a combination of stimulants and sedatives routinely prescribed by psychiatrists to help patients manage conditions like anxiety and ADHD.
The White House, federal agencies and lawmakers recently marked the elimination of the DATA-Waiver Program, better known as the X-Waiver requirement, with calls for providers to begin incorporating opioid use disorder treatment buprenorphine in everyday patient care.
The X-Waiver requirement only permitted doctors who had received specialized training and federal permissions to prescribe the opioid partial agonist, which is a controlled substance.
A New York law aims to ensure that naloxone is available if needed by people who take prescription opioids.
Under the law, which took effect last summer, doctors must co-prescribe naloxone to certain patients who are at risk of an overdose when writing the patients’ first opioid prescription each year. Risk factors that would trigger the requirement include taking a high daily dose of an opioid (at least 90 morphine milligram equivalents, or MME); taking certain other drugs, like sedative hypnotics; or having a history of substance use disorder. At least 10 other states have similar laws, according to research by the Network for Public Health Law.
A former Tennessee correctional officer will receive $160,000 in back pay and damages after he was forced to resign for taking Suboxone to treat his opioid use disorder, if a judge approves a landmark consent decree filed in federal court in Nashville.
It is the first time the U.S. Department of Justice has used the Americans with Disabilities Act to settle a claim that an employee was discriminated against for taking a prescribed medication to treat drug addiction, according to the Department.
The opioid epidemic has caused thousands of deaths in the United States over the years, but a new study shows that more patients are shifting to medical cannabis to treat their chronic pain.
Published in the JAMA Network Open last week, the study shows that 1 in 4 adults (25.9%) with chronic pain is turning to cannabis.
The authors of this study have surveyed 1,724 adults aged 18 years or older with chronic pain who lived in the 36 U.S. states and Washington, DC that have a medical cannabis program.

THE OTHER SUPREME COURT RULING WITH BIG REPERCUSSIONS FOR U.S. HEALTH CARE
This month's Comment Section looks specifically at a recent Supreme Court ruling that has significant impact on how healthcare providers can and will prescribe opioid-based medications to their patients.
Just three days after the thunderous ruling in Dobbs v. Jackson Women’s Health Organization, the Supreme Court issued another important decision on a pressing healthcare issue. It’s a seemingly technical opinion interpreting a single phrase of a federal law on the prescription of controlled substances, and it garnered little attention amid the court’s end-of-term Sturm und Drang. But eight months later, it is already reshaping the government’s response to America’s overdose crisis.
The case, Ruan v. United States, involved the criminal convictions of two doctors who were accused of running opioid “pill mills.” The Controlled Substances Act makes it a crime to prescribe opioids unless the prescription is “authorized.” A federal regulation, in turn, says a prescription is “authorized” only if a doctor writes it for a “legitimate medical purpose” while “acting in the usual course of his professional practice.”


NORCAL TRANSACTION CERTIFICATE HOLDER UPDATE
PLEASE HELP US REMIND YOUR CLIENTS TO REVIEW THEIR
CONTACT INFORMATION WITH COMPUTERSHARE
PROASSURANCE WELCOMES TORI WARSKO AND ANTHONY PETERSON
We are pleased to announce that Tori Warsko has joined ProAssurance as Business Development Representative for the Southwest Region, effective January 9, 2023.
Tori comes to us from Wolverine Mutual Insurance Company where she acted as Territory Manager for the entire state of Indiana and the southwest region of Michigan. She has extensive experience working with principals and producers to identify business obstacles, establish financial goals, and tailor products to individual markets. She earned her bachelor’s degree in Business Administration from Ferris State University.
You can reach Tori directly at 205-776-3066 or ToriWarsko@ProAssurance.com.
.png?width=300&name=MicrosoftTeams-image%20(13).png)
We are pleased to welcome Anthony (Tony) Peterson to ProAssurance, where he will serve as Assistant Vice President of Business Development for the Southeast Region, effective January 30, 2023.
Tony has more than 30 years of MPL industry experience and is skilled in developing and managing complex risk and insurance programs. He comes to us from MagMutual Insurance Company, where he was Vice President of Business Development and responsible for the growth and retention strategy for hospitals and healthcare systems across the U.S. He has extensive strategic leadership experience and a talent for developing and maintaining trusted relationships with key agent/broker partners. He attended DePaul University, College of Business, and holds the Associate in Risk Management (ARM) designation from the American Insurance Institute.
You can reach Tony directly at AnthonyPeterson@ProAssurance.com.
.png?width=300&name=MicrosoftTeams-image%20(12).png)
PLEASE JOIN US IN WELCOMING TORI AND TONY TO PROASSURANCE AND WISHING THEM SUCCESS IN THEIR NEW ROLES.










.png?width=300&name=MicrosoftTeams-image%20(39).png)